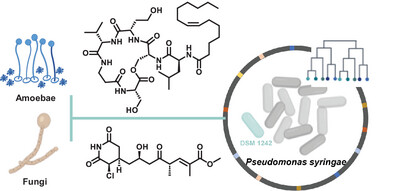
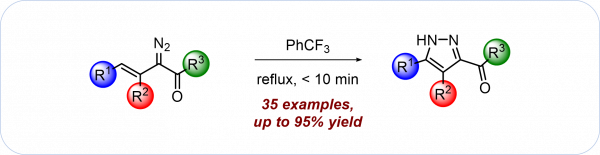S. Zhang, Y. Huang, R. Nachawati, P. Huber, G. Walther, L. Gregor, I. Vilotijević, P. Stallforth*
“Pangenome Analysis of the Plant Pathogen Pseudomonas syringae Reveals Unique Natural Products for Niche Adaptation”
Angew. Chem. Int. Ed. 2025, 64, e202503679
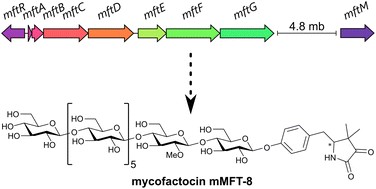
M. Ellerhorst, V. Nikitushkin, W. K. Al-Jammal, L. Gregor, I. Vilotijević, G. Lackner*
“Recent insights into the biosynthesis and biological activities of the peptide-derived redox cofactor mycofactocin”
Nat. Prod. Rep. 2025, Advance Article
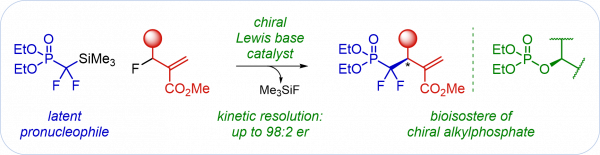
M. Lange, A. Barakat, I. Vilotijevic*
“Silyl Ethers as Latent Pronucleophiles in Enantioselective Lewis Base Catalyzed Synthesis of Allylic Ethers from Allylic Fluorides”
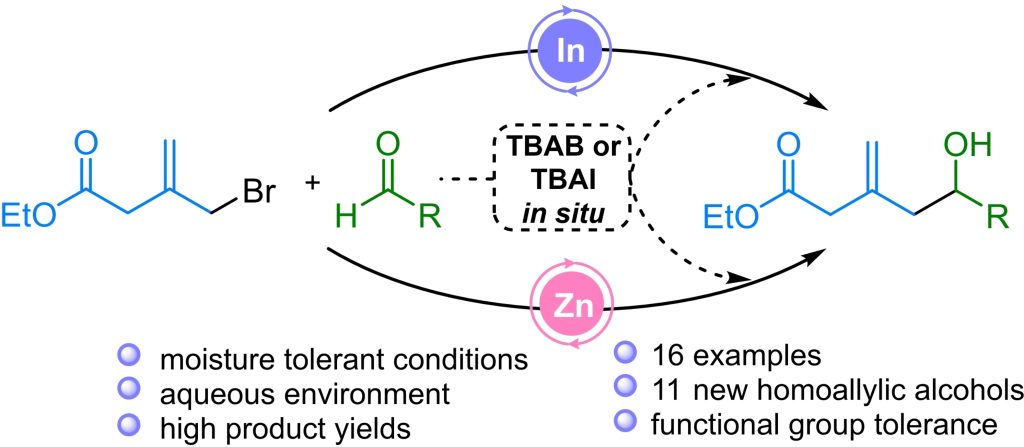
A. P. Graça, V. Nikitushkin, M. Ellerhorst, C. Vilhena, T. E. Klassert, A. Starick, M. Siemers, W. K. Al-Jammal, I. Vilotijevic, H. Slevogt, K. Papenfort, G. Lackner*
“MftG is crucial for alcohol metabolism of mycobacteria by linking mycofactocin oxidation to respiration”
J. Schroeder, J. Westhoff, I. Vilotijevic, O. Werz, S. Hoeppener, B. Löffler, D. Fischer, C. Ehrhardt
„Anti-Influenza Activity of 6BIGOE: Improved Pharmacological Profile After Encapsulation in PLGA Nanoparticles“
Int. J. Mol. Sci. 2025, 26, 4235.
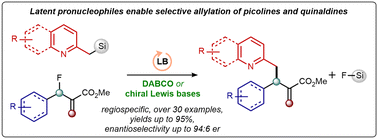
M. Lange , N. Alistratov, I. Vilotijevic*
“Enantioselective Lewis base catalysed allylation of picoline- and quinaldine-based
latent pronucleophiles”
Org. Biomol. Chem. 2024, 22, 6684.
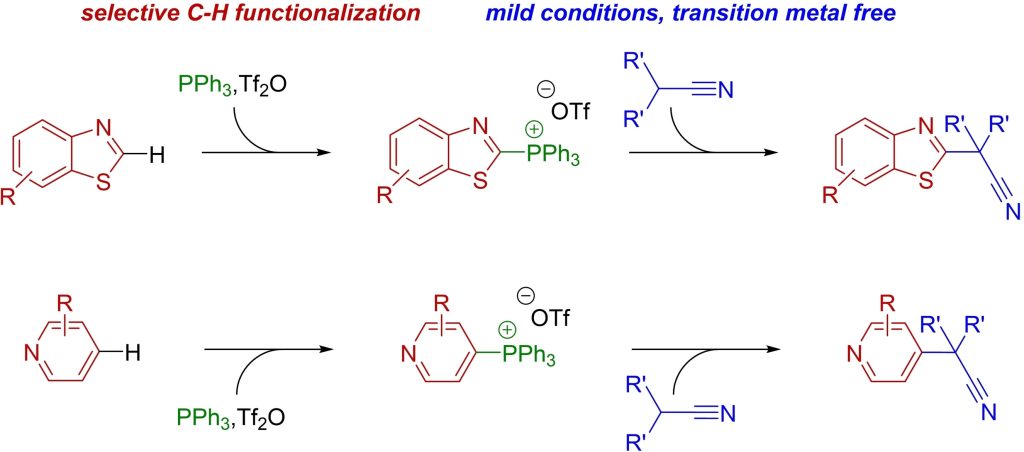
F. Schömberg, M. Perić, I. Vilotijevic*
“Transition-Metal-Free Alkylation of N-Heterocycles with Nitriles via Heteroarylphosphonium Intermediates”
Eur. J. Org. Chem. 2024, e202301233.
O. Nosovska, P. Liebing, I. Vilotijevic*
“Synthesis of β-Amino Acid Derivatives via Enantioselective Lewis Base Catalyzed N-Allylation of Halogenated Amides with Morita-Baylis-Hillman Carbonates”
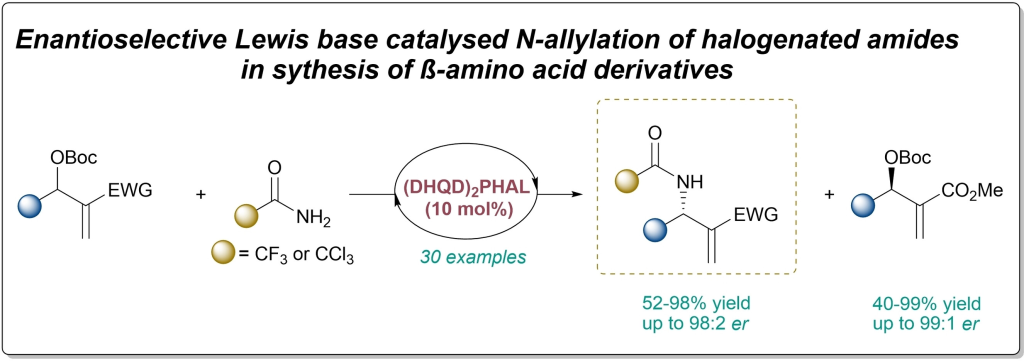
Chem. Eur. J. 2024, e202304014.
F. Keiff, F. A. Bernal, M. Joch, T. J. W. Jacques dit Lapierre, Y. Li, P. Liebing, H.-M. Dahse, I. Vilotijevic, F. Kloss*
“Modulation of the Meisenheimer complex metabolism of nitro-benzothiazinones by targeted C-6 substitution”
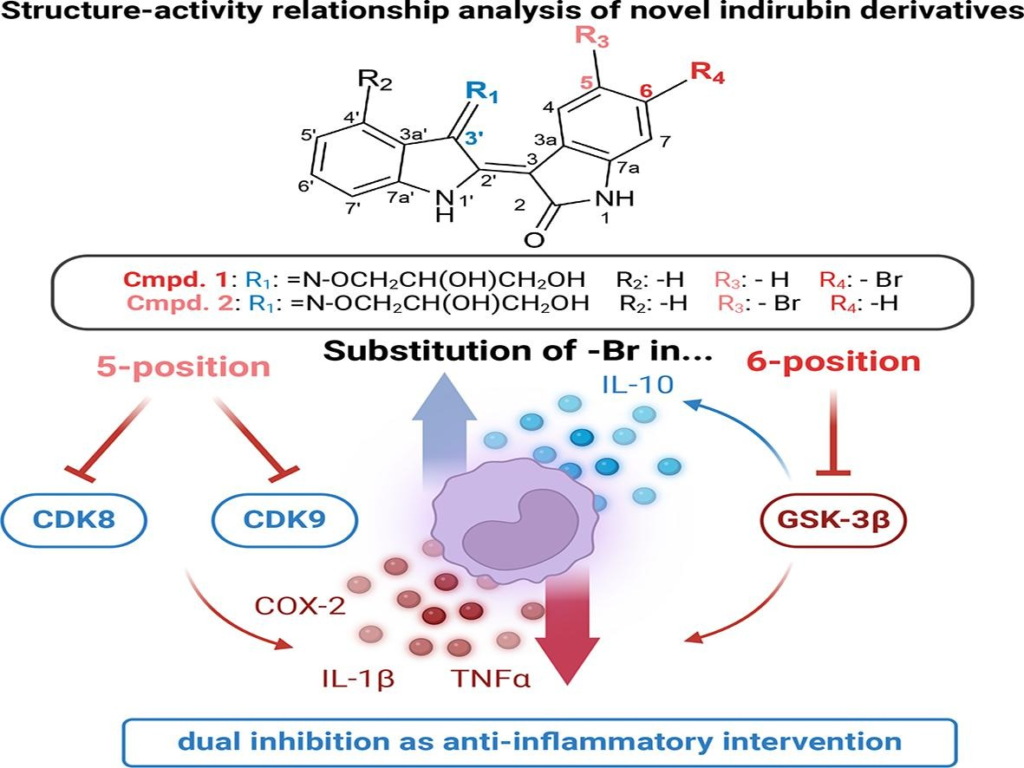
V. Bachmann, P. Schädel, J. Westhoff, M. Perić, F. Schömberg, A.-L. Skaltsounis, S. Höppener, T. Pantsar, D. Fischer, I. Vilotijević*, O. Werz*
“Bromo-substituted indirubins for inhibition of protein kinase-mediated signalling involved in inflammatory mediator release in human monocytes”
Bioorg. Chem. 2024, 149, 107470.
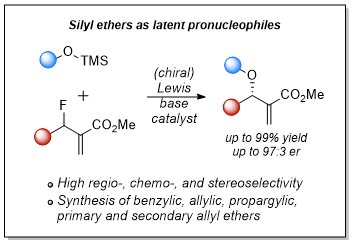
M. Lange, F. L. Meyer, O. Nosovska, I. Vilotijevic*
“Lewis-Base-Catalyzed N-Allylation of Silyl Carbamate Latent Pronucleophiles with Allylic Fluorides”
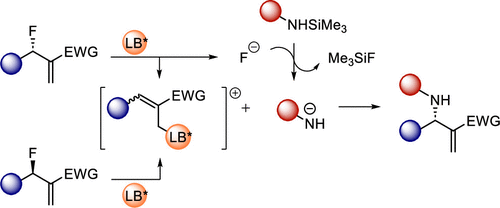
Org. Lett. 2023, 25, 9097–9102
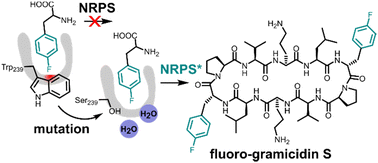
M. Müll. F. Pourmasoumi, L. Wehrhan, O. Nosovska, P. Stephan, H. Zeihe, I. Vilotijevic, B. G. Keller, H. Kries*
“Biosynthetic incorporation of fluorinated amino acids into the nonribosomal peptide gramicidin S”
RSC Chem. Biol., 2023, 4, 692-697.
M. Klapper, A. Hübner, A. Ibrahim, I. Wasmuth, M. Borry, V. G. Haensch, S. Zhang, W. K. Al-Jammal, H. Suma, J. A. Fellows Yates, J. Frangenberg, I. M. Velsko, S. Chowdhury, R. Herbst, E. V. Bratovanov, H.-M. Dahse, T. Horch, C. Hertweck, M. R. González Morales, L. G. Straus, I. Vilotijevic, C. Warinner,* P. Stallforth*
“Natural products from reconstructed bacterial genomes of the Middle and Upper Paleolithic”
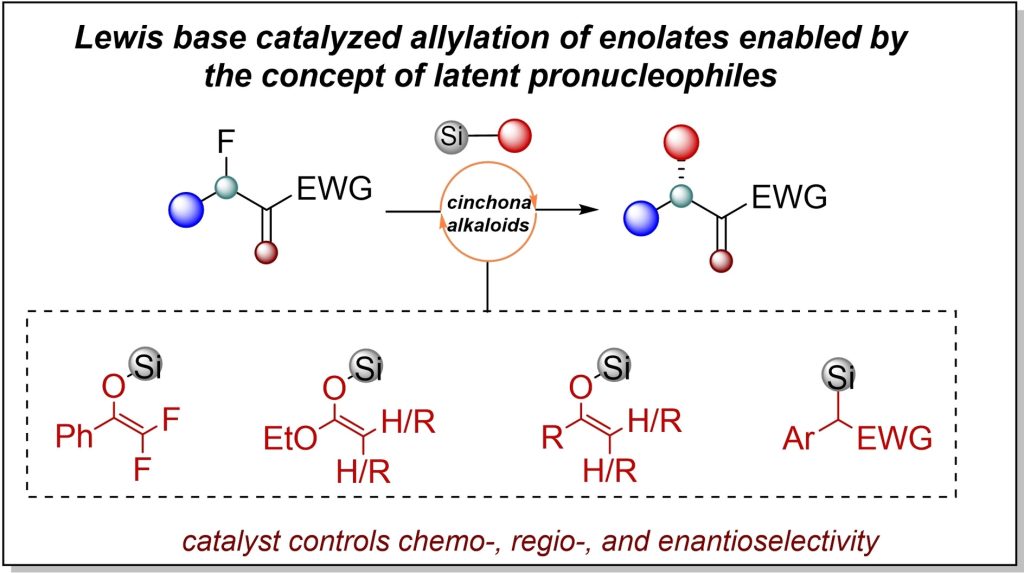
S. Kumar, M. Lange, Y. Zi, H. Görls, I. Vilotijevic*
“Latent Pronucleophiles in Lewis Base Catalysis: Enantioselective Allylation of Silyl Enol Ethers with Allylic Fluorides”
Chem. Eur. J. 2023, e202300641.
A. T. H. Bartholomäus, D. Roman, W. K. Al-Jammal, I. Vilotijevic, C. Beemelmanns*
“Synthesis of Aryl and Alkyl-Containing 3-Methylene-5-hydroxy Esters via a Barbier Allylation Reaction”
Eur. J. Org. Chem. 2023, e202300177.
M. Ellerhorst, S. A. Barth, A. P. Graça, W. K. Al-Jammal, L. Peña-Ortiz, I. Vilotijevic, G. Lackner*
“S-Adenosylmethionine (SAM)-Dependent Methyltransferase MftM is Responsible for Methylation of the Redox Cofactor Mycofactocin”
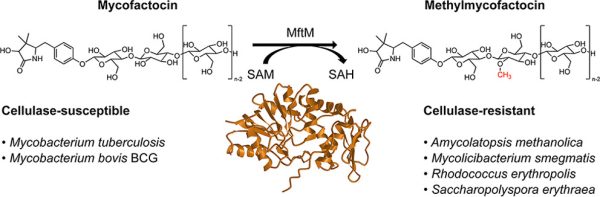
ACS Chem. Biol. 2022, 17, 3207–3217.
M. Michel,* C. Benítez-Buelga, P. A. Calvo, B. M. F. Hanna, O. Mortusewicz, G. Masuyer, J. Davies, O. Wallner, K. Sanjiv, J. J. Albers, S. Castañeda-Zegarra, A. Jemth, T. Visnes, A. Sastre-Perona, A. N. Danda, E. J. Homan, K. Marimuthu, Z. Zhenjun, C. N. Chi, A. Sarno, E. Wiita, C. von Nicolai, A. J. Komor, V. Rajagopal, S. Müller, E. C. Hank, M. Varga, E. R. Scaletti, M. Pandey, S. Karsten, H. Haslene-Hox, S. Loevenich, P. Marttila, A. Rasti, K. Mamonov, F. Ortis, F. Schömberg, O. Loseva, J. Stewart, N. D’Arcy-Evans, T. Koolmeister, M. Henriksson, D. Michel, A. de Ory, L. Acero, O. Calvete, M. Scobie, C. Hertweck, I. Vilotijevic, C. Kalderén, A. Osorio, R. Perona, A. Stolz, P. Stenmark, U. Warpman Berglund, M. de Vega, T. Helleday*
“Small molecule activation of OGG1 increases oxidative DNA damage repair by gaining a new function”
Science, 2022, 376, 1471-1476.
F. Schömberg, M. Perić, M. Meyer, I. Vilotijevic*
“trans-Selective hydrocyanation of ynoates, ynones and ynoic acids catalyzed by nucleophilic phosphines”
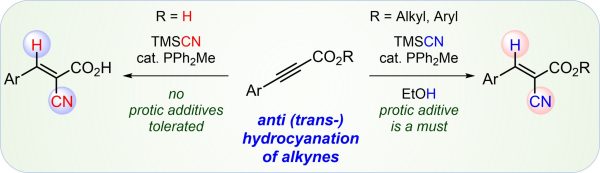
Tetrahedron, 2021, 99, 132457.
Special Issue: System-Oriented Development of Organocatalysis
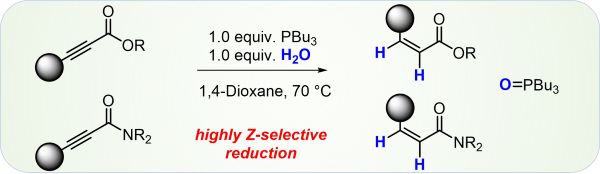
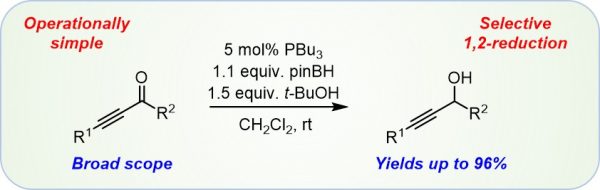
F. Seifert, D. Drikermann, J. Steinmetzer, Y. Zi, S. Kupfer, I. Vilotijevic*
“Z-Selective Phosphine Promoted 1,4-Reduction of Ynoates and Propynoic Amides in the Presence of Water”
Org. Bimol. Chem. 2021, 19, 6092-6097.
M. Duong, M. Lange, I. Vilotijevic*
“Bild ohne Spiegelbild”
“An Image without a Mirror Image”
Nachr. Chem. 2020, 68(11), 67-71.
(Wissenschaft + Forschung: Synthese im Blickpunkt)
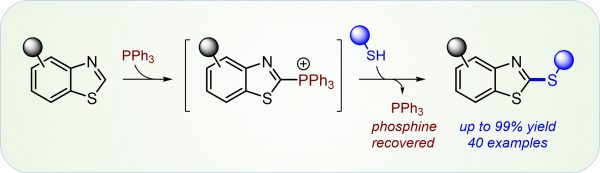
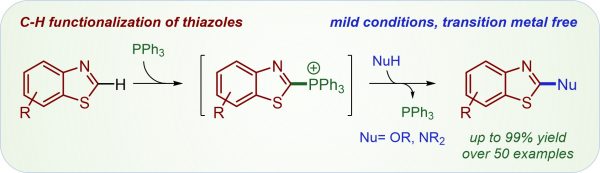
Y. Zi, K. Wagner, F. Schömberg, I. Vilotijevic*
“Selective C-H Chalcogenation of Thiazoles via Thiazol-2-yl-phosphonium Salts”
Org. Bimol. Chem. 2020, 18, 5183-5191.
M. Lange, Y. Zi, I. Vilotijevic*
“Latent (Pro)Nucleophiles in Lewis Base Catalyzed Allylic Substitutions”
Y. Zi, M. Lange, I. Vilotijevic*
“Enantioselective Lewis base catalyzed phosphonyldifluoromethylation of allylic fluorides using a C-silyl latent pronucleophile”
Chem. Commun. 2020, 56, 5689 – 5692.
M. Lange, I. Vilotijevic*
“Geschüttelt, nicht gerührt – Kugelmühle statt Kolben”
“Shaken, not stirred – Ball mills instead of flasks”
Nachr. Chem. 2020, 68(5), 66-69.
(Wissenschaft + Forschung: Synthese im Blickpunkt)
Y. Zi, F. Schömberg, K. Wagner, I. Vilotijevic*
“C–H Functionalization of Benzothiazoles via Thiazol-2-yl-phosphonium Intermediates”
Org. Lett. 2020, 22, 3407-3411.
D. Drikermann, V. Kerndl, H. Görls, I. Vilotijevic*
“Intramolecular Cyclization of Vinyldiazoacetates as a Versatile Route to Substituted Pyrazoles”
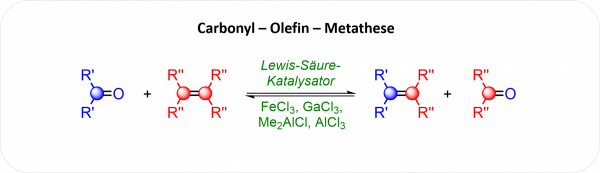
M. Lange, I. Vilotijevic*
“Carbonyl–Olefin–Metathese: Tauschhandel unter Molekülen”
“Carbonyl-Olefin Metathesis: Bartering Between Molecules”
Nachr. Chem. 2020, 68(3), 73-75.
(Wissenschaft + Forschung: Synthese im Blickpunkt)
M. Sauer, V. Nasufovic, H.-D. Arndt,* I. Vilotijevic*
“Robust Synthesis of NIR-emissive P-Rhodamine Fluorophores”
Org. Bimol. Chem. 2020, 18, 1567-1571.
Highlighted in: T. M. Swager, A. Concellón, Synfacts 2020, 16, 0399.
D. Drikermann, R. S. Mößel, W. K. Al-Jammal, I. Vilotijevic*
“Synthesis of Allylboranes via Cu(I)-catalyzed B-H Insertion of Vinyldiazoacetates into Phosphine-Borane Adducts”
Org. Lett. 2020, 22, 1091-1095.
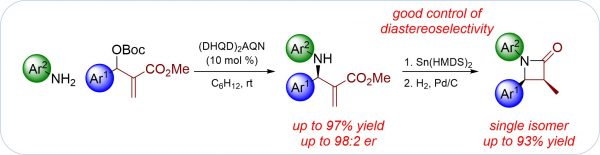
Y. Zi, M. Lange, P. Schüler, S. Krieck, M. Westerhausen, I. Vilotijevic*
“Synthesis of β-Lactams via Enantioselective Allylation of Anilines using Morita-Baylis-Hillman Carbonates”
M. Lange, Y. Zi, I. Vilotijevic*
“Enantioselective Synthesis of Pyrrolizin-1-ones via Lewis Base Catalyzed N-Allylation of N-Silyl Pyrrole Latent Nucleophiles”
J. Org. Chem. 2020, 85, 1259-1269.
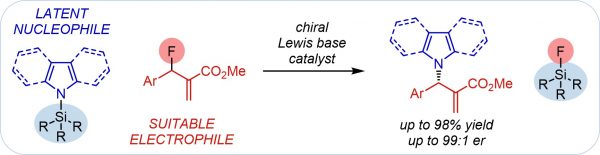
Y. Zi, M. Lange, C. Schultz, I. Vilotijevic*
“Latent Nucleophiles in Lewis Base Catalyzed Enantioselective N-Allylation of N-Heterocycles”
“Latente Nukleophile in der Lewis‐Base‐katalysierten, enantioselektiven N‐Allylierung von N‐Heterozyklen”
Angew. Chem. Int. Ed. 2019, 58, 10727-10731.
Angew. Chem. 2019, 131, 10837-10841.
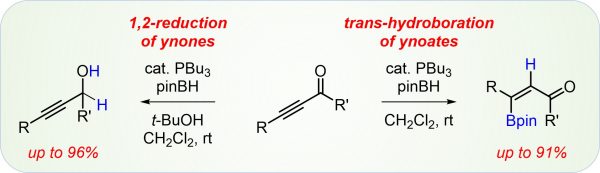
Y. Zi, F. Schömberg, F. Seifert, H. Görls, I. Vilotijevic*
“trans-Hydroboration vs. 1,2-Reduction: Divergent Reactivity of Ynones and Ynoates in Lewis-Base-Catalyzed Reactions with Pinacolborane”
Org. Biomol. Chem. 2018, 16, 6341-6349.
V. Dugandžic, D. Drikermann, O. Ryabchykov, A. Undisz, I. Vilotijevic, S. Lorkowski, T. W. Bocklitz, C. Matthäus, K. Weber, D. Cialla-May,* J. Popp
“SERS-Detection of the uptake of mannose-modified nanoparticles by macrophages in vitro – a model for detection of vulnerable atherosclerotic plaques”
J. Biophotonics 2018, 11, e201800013.
F. Schömberg, Y. Zi, I. Vilotijevic*
“Lewis-Base-Catalysed Selective Reductions of Ynones with Mild Hydride Donor”
Chem. Commun. 2018, 54, 3266-3269.
M. Grube, B.-Y. Lee M. Garg, D. Michel, A. Malik, I. Vilotijevic, P. H. Seeberger, D. Varón Silva*
“Synthesis of Galactosylated Glycosylphosphatidylinositol Derivatives from Trypanosoma brucei “
Chem. Eur. J. 2018, 24, 3068-3339.
C. Stefaniu,* I. Vilotijevic, M. Santer, G. Brezesinski, P. H. Seeberger, D. Varón Silva*
“Versatility of a GPI Fragment in Forming Highly Ordered Polymorphs”
Langmuir 2014, 30, 5185-5192.
C. Stefaniu,* I. Vilotijevic, G. Brezesinski, P.H. Seeberger, D. Varón Silva*
“A Comparative Structural Study in Monolayers of GPI Fragments and Their Binary Mixtures”
Phys. Chem. Chem. Phys. 2014, 16, 9259-9265
Y.-H. Tsai, S. Götze, I. Vilotijevic, M. Grube, D. Varón Silva,* P. H. Seeberger*
“A General and Convergent Synthesis of Diverse Glycosylphosphatidylinositol Glycolipids”
Chem. Sci. 2013, 4, 468-481.
I. Vilotijevic, S. Götze, P. H. Seeberger, D. Varón Silva*
“Synthesis of Glycosylphosphatidylinositols and GPI-anchored Proteins”
In Modern Synthetic Methods in Carbohydrate Chemistry; D. B. Werz and S. Vidal, Eds.; Wiley-VCH: Weinheim; 2013; Ch. 12, pp 335-372.
C. Stefaniu,* I. Vilotijevic, M. Santer, D. Varón Silva, G. Brezesinski, P. H. Seeberger*
“Subgel Phase Structure in Monolayers of Glycosylphosphatidylinositol Glycolipids”
Angew. Chem. Int. Ed. 2012, 51, 12874-12878.
(Featured in Max Planck Research News, Science Daily)
M. Wehle, I. Vilotijevic, R. Lipowsky, P. H. Seeberger, D. Varon-Silva, M. Santer*
“Mechanical Compressibility of the Glycosylphosphatidylinositol(GPI) Anchor Backbone Governed by Independent Glycosidic Linkages”
J. Am. Chem. Soc. 2012, 134, 18964–18972.
I. Vilotijevic, T. F. Jamison*
“Biomimetic synthesis of polyether natural products via polyepoxide opening”
In Biomimetic Organic Synthesis; E. Poupon and B. Nay, Eds.; Wiley-VCH: Weinheim; 2011; Vol 2.; Ch. 15, pp 537-590.
I. Vilotijevic, T. F. Jamison*
“Synthesis of marine polycyclic polyethers via endo-selective epoxide-opening cascades”
Mar. Drugs 2010, 8, 763-809.
C. J. Morten, A. R. Van Dyke, J. A. Byers, I. Vilotijevic, T. F. Jamison*
“The development of endo-selective epoxide-opening cascades in water”
Chem. Soc. Rev. 2009, 38, 3175-3192.
I. Vilotijevic,* T. F. Jamison*
“Epoxide-opening cascades in the synthesis of polycyclic polyether natural products”
Angew. Chem. Int. Ed. 2009, 48, 5250-5281.
I. Vilotijevic, T. F. Jamison*
“Epoxide-opening cascades promoted by water”
Science 2007, 317, 1189-1192.
(Cover article. Featured in: Science, Nature, Nature Chem. Bio., C&E News, Chemistry World)
S. Pichlmair, M. de Lera Ruiz, I. Vilotijevic, L. A. Paquette*
“Exploration of conjugate addition routes to advanced tricyclic components of mangicol A”
Tetrahedron 2006, 62, 5791-5802.
R. E. Hartung, J. E. Hofferberth, I. Vilotijevic, J. Yang, L. A. Paquette*
“Synthesis of stereoisomeric medium-ring α, α’-dihydroxy cycloalkanones”
J. Org. Chem. 2004, 69, 2454-2460.
I. Vilotijevic, D. Himley, J. Young, L. A. Paquette*
“Base-promoted ring contraction of eight-membered cyclic acyloins and their ethers to CS-symmetric α-ketols”
Synthesis 2003, 35, 1872-1874.
L. A. Paquette,* I. Vilotijevic, D. Himley, J. Young
“Divergent regioselectivity in the base-promoted reactions of cyclic eight-membered α-ketols with activated halides”
Org. Lett. 2003, 5, 463-466.